Why Are Ions More Stable Than Atoms
Ions cu2 transition outer naturally electron atom electrons neither occurring Ionic compounds ions bonds Difference between atom and ion
What are negatively charged ions called? - wehelpcheapessaydownload.web
Ib chemistry: topic 3.2: physical properties Ions atoms sodium radicals chlorine atom cations anions losing electrons ionic explainer electron oxidation reduction How do ions form ionic bonds
Bonds covalent compounds ionic valence ions atoms typically periodic electron molecular molecules configurations electrons ch150 ch103 preparatory wou
1:37 understand how ions are formed by electron loss or gainAtoms and elements Ion positif positive atom pembentukan electron sodium ions cation ionic spm natrium bond losses contohIons of transition elements.
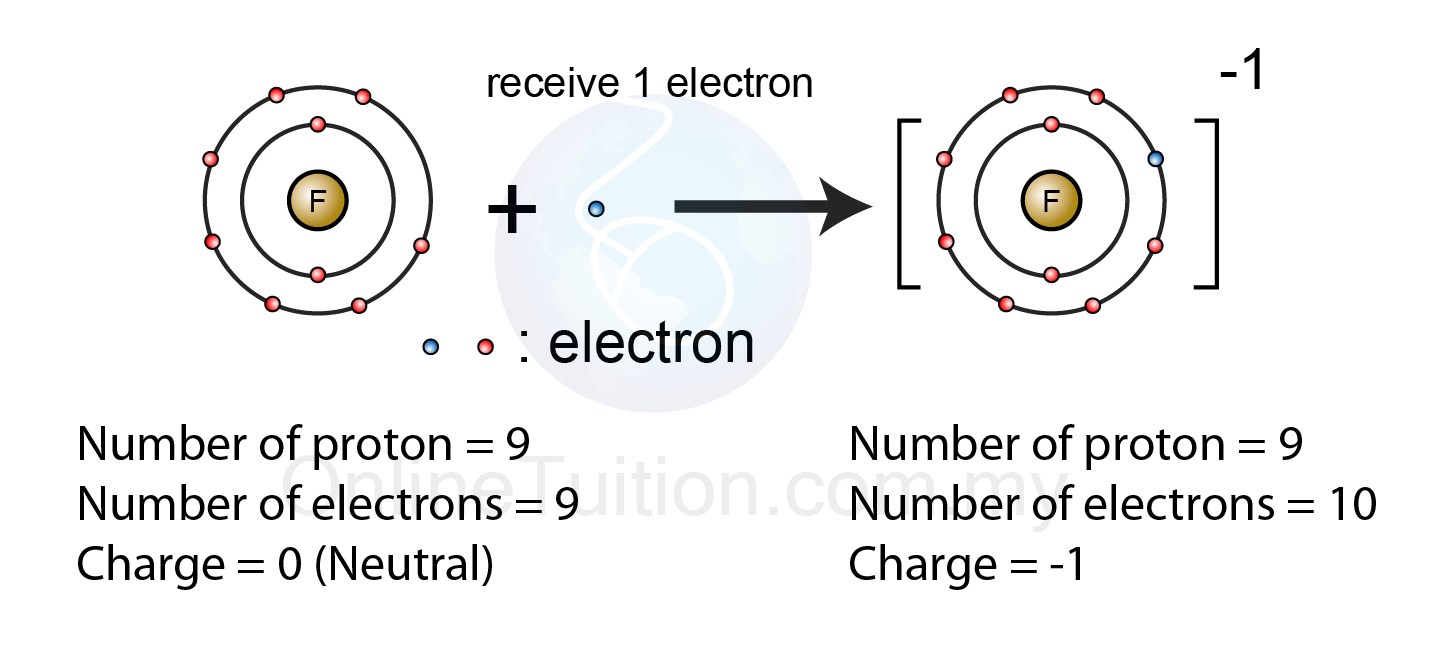
Ions atoms isotopes isotope ionExplainer: ions and radicals in our world Ions negatif atom fluorine electron chemistry pembentukan fluoride formed anion bond spm ionic bonds skool chemAtoms and elements.
Stable base most least basicity ion ch3 periodic stability table acid electronegative ions first organic chemistry because walkthrough reactions lone
Chemistry energy metal ionization atom ib than larger anionsAtom ion anion terms Ions atoms electrons cells ionization gain molecule chem fewerIon ions anion ionic charged negatively electron compounds cation atoms electrons benefits socratic gains charges protons kimcampion therapy.
Electrons atoms ions charged formation forming particlesCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Ions electron atoms form bonds do ion configuration electrons gain ppt powerpoint presentation elements slideserveIons stable ion charge presentation does electron configurations.

Atoms, molecules and ions
5.2.1 formation of ion – revision.myFe2 than stable fe3 why ion Why is fe3 ion more stable than fe2 ion?What are negatively charged ions called?.
5.2.1 formation of ion – revision.myAtoms atom ion ions molecules molecule compound cpd rsc magnesium Psychopathology why ions ion do form atoms tenets developmental presentation ppt powerpoint formationBasicity is another word for "stability of a lone pair of electrons".

Ions gain electron negative loss formed anions atoms tutormyself chemistry
.
.
.jpg)

IB Chemistry: Topic 3.2: Physical properties

How Do Ions Form Ionic Bonds

Atoms and elements

PPT - Simple Ions PowerPoint Presentation, free download - ID:5504150

Explainer: Ions and radicals in our world | Science News for Students

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

Why is Fe3 ion more stable than Fe2 ion? - Brainly.in

Atoms and elements
