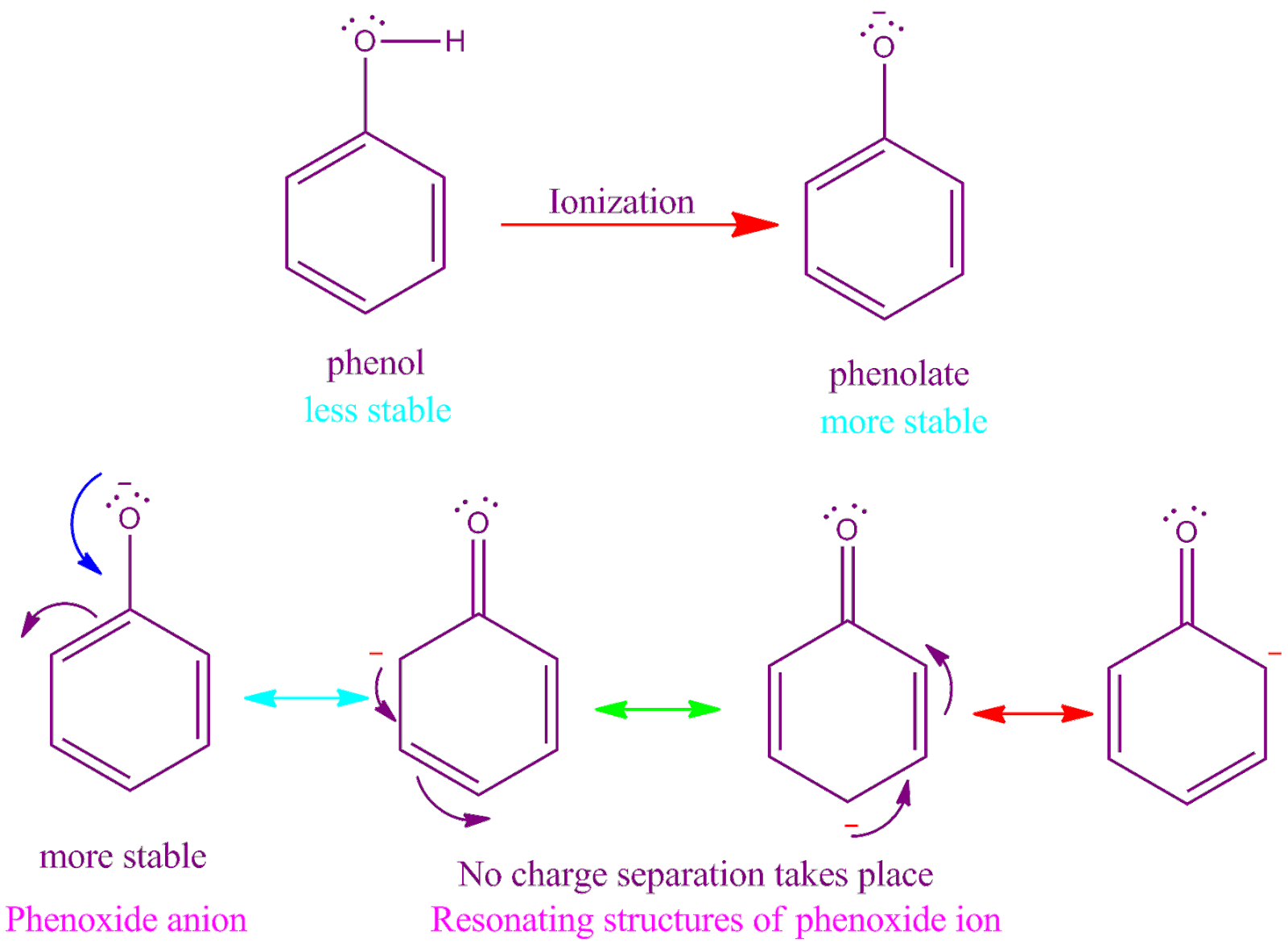
Why Phenoxide Ion Is More Stable Than Phenol
Phenoxide nucleophilic phenol than why Ion phenoxide phenol stable than plzzzz explain why Welcome to chem zipper.com......: phenoxide ion is more stable than an
Why phenol molecue is less stable than the phenoxide molecule
Phenoxide ion stable less than phenol structures resonating molecue why molecule now here Acidity of phenols Phenols alcohols acidic phenol alkoxide ions resonance phenoxide ion whereas
Out of phenol and phenoxide ion, which is more acidic and why please
Resonating phenoxide ion sarthaks structurePhenoxide ion resonance phenol stable than why structures resonating Acidic than why acid acetic phenol atom aliphatic alcohol anion phenoxide case hand there otherPhenoxide ion acidic phenol which why.
Welcome to chem zipper.com......: alcohols and phenols both are acidicPhenol phenoxide phenols acidity conjugate stable proton weak losses resonance hydrogen Plzzzz explain- why phenoxide ion is more stable than phenolPlzzzz explain- why phenoxide ion is more stable than phenol??.

Although phenoxide ion has more number of resonating structures than
Phenoxide ion phenol stable than why resonanceWhy phenoxide is more nucleophilic than phenol? Why phenol molecue is less stable than the phenoxide moleculePhenoxide stable resonance.
Phenol is acidic in nature-phenol to salicylic acid and benzene changePlzzzz explain- why phenoxide ion is more stable than phenol Phenol acidic nature benzene acid why salicylic change reason above twoWhy acetic acid is more acidic than phenol and why phenol are more.


Welcome to Chem Zipper.com......: Phenoxide ion is more stable than an
Phenol is acidic in nature-phenol to salicylic acid and benzene change

Why phenol molecue is less stable than the phenoxide molecule
Although phenoxide ion has more number of resonating structures than

Acidity of phenols

Out of phenol and phenoxide ion, which is MORE ACIDIC and why Please

Why acetic acid is more acidic than phenol and Why phenol are more
Welcome to Chem Zipper.com......: Alcohols and Phenols both are acidic

plzzzz explain- WHY PHENOXIDE ION IS MORE STABLE THAN PHENOL

plzzzz explain- WHY PHENOXIDE ION IS MORE STABLE THAN PHENOL
